
- Links
- Science China
- Analytical Chemistry
- Science
- Nature
- SCI
- ACS Journals
- RSC Journals
- Wiley InterScience
- Elesiver
- Springer Link
- Related Links
- Symposium for Cell Analysis on Micro/Nanofluidics
- 2019 China-Japan-Korea Symposium on Analytical Chemistry
- IUPAC International Congress on Analytical Chemistry (Hainan)
- International Symposium on Luminescence Spectrometry
- 国家留学网
- 中国化学会
- 清华大学化学系
Page views:1395719
Based on the microfluidic chip coupled with ESI-Q-TOF mass spectrometry cells co-cultured and cell communication research
Based on the accumulation of cell research and build a microfluidic chip-mass spectrometry platform, a microfluidic device was integrated in a controlled coculture system, in which the secreted proteins were qualitatively and semi-quantitatively determined by a directly coupled mass spectrometer. PC12 cells and GH3 cells were cocultured under various conditions as a model of the regulation of the organism by the nervous system. A micro-solid phase extraction (SPE) column was integrated in order to remove salts from the cells secretion prior to mass spectrometry detection. A three layer polydimethylsiloxane (PDMS) microfluidic device was fabricated to integrate valves for avoiding contamination between the cells coculture zone and the pretreatment zone. Electrospray ionization (ESI)-quadrupole (Q)-time of flight (TOF)-mass spectrometry was employed to realize highly sensitive qualitative analysis and to implement semiquantitative analysis. Furthermore, cell migrations under various coculture conditions were observed and discussed. The inhibition on growth hormone secretion from GH3 cells by dopamine released from PC12 cells was investigated and demonstrated. Thus, the developed platform provides a useful tool on cell to cell signaling studies for disease monitoring and drug delivery control.
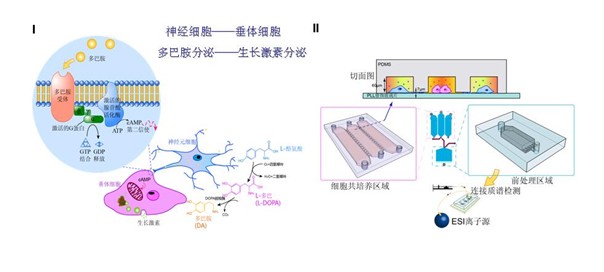
Scheme shows the synthesis of dopamine as one of the neurotransmitters in the neuron cell. The growth hormone release behavior of pituitary cells was regulated as a function of neurotransmitters.
The student Wei huibin developed a microfluidic device which was coupled with mass spectrometry for various types of cell cocultures and signal factor analysis. A micro-SPE column was integrated into the chip in order to remove salts from samples obtained in biological environments, which is necessary for ESI-MS detection. This platform has proven to be a versatile and powerful tool to study cell signaling for various biological applications. It provides a well-controlled cell culture environment which can be adjusted more precisely by fabricating condition control structures. This combined system allows multiple biochemical factors, essential in mimicking physiological conditions as cells constantly receive signals from soluble environments. Furthermore, the character and content of the released factor in the regulation pathway was determined by the highly sensitive MS. This cell coculture platform would be very useful in modeling cancer progression and testing therapeutics in a biologically relevant context. The known and unknown essential signaling factors would be studied in the important regulation pathways for disease monitoring and drug delivery control. We are planning to apply the present technique to the perivascular epithelioid cells and liver cells coculture to investigate their signaling pathway and generate a physiologically relevant in vitro model. The research results were published in Analytical Chemistry.
Production of integrated microfluidic chip porous membrane structure for cell screening and separation
Cooperation with the American Academy of Sciences, the Chinese Academy of foreign academicians, Richard N. Zare, Stanford University professor, we demonstrate a proof-of-concept of this device by fabricating a particle sorter that is capable of separating poly-styrene microbeads of different sizes (2.5 mmto20 mm), as well as different components of whole blood. The flow first passes through the larger pores before it passes through the smaller pores of the same membrane. For polystyrene microbeads, greater than 99.9% separation efficiency was achieved, whereas for whole blood 99.7% separation efficiency was demonstrated. We believe the simplicity of this approach makes it quite appealing, especially for being integrated into point-of-care diagnostics. The research results were published in Lab on a Chip.
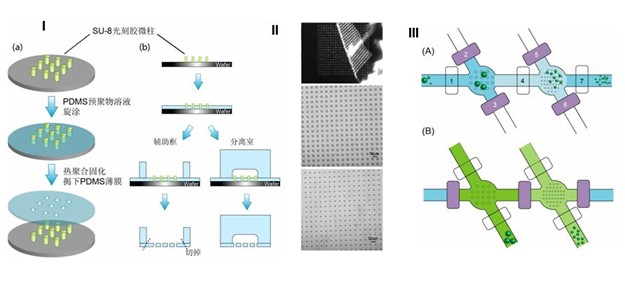
Left: (A) Schematic of the procedure for making a porous PDMS membrane from the POM-mold. Illustration of the cross-section is presented on the right. (B) Image of the porous PDMS membrane being peeled from the POM-mold. Right: Procedure for sorting particles with three different diameters. The darker color represents liquid flow in the bottom layer; the lighter color represents liquid flow in the top flow layer.
We have described a microfluidic particle sorter which incorporates a PDMS porous membrane. Membranes containing pores as small as 6.4 mm were easily fabricated on a POM-mold, and even smaller pores were generated by aligning two or more membranes so that the pores overlapped. We were able to separate white blood cells, as well as red blood cells and platelets combined, from whole blood. Additionally, the size of the pores could be conveniently changed to fit the specific objective, such as circulating tumor cells (CTCs). We believe that these results demonstrate the potential of this simple monolithic microfluidic particle sorter to be integrated in miniaturized and automatic equipment for point-of-care diagnostics. Further work is needed to perfect this setup for isolation, collection, and enumeration of blood components, but the present results clearly demonstrate the principles of this new separation device.